The research has been implemented within the framework of the project “Bacterial microcompartments as synthetic bioreactors”. The leading researcher of UL Institute of Microbiology and Biotechnology Dr.biol. Jānis Liepiņš is the project manager. With the administrative support of the UL Foundation, the successful course of the project was ensured by a donation from the patron “Mikrotīkls”.
Simple organisms with broad functionality
Bacteria are relatively “simple” organisms, their cells do not contain many of the structures distinctive to plant or animal cells, including the nucleus, mitochondria and chloroplasts. However, bacterial cell sometimes form separate compartments, which, under certain conditions, significantly facilitate the course of important chemical reactions – microcompartments. These are groups of enzymes that are encapsulated in a protein shell, which together perform an important function in the body, such as carbon fixation or the cleavage of certain substances. Depending on the encapsulated enzymes, about 30 different types of bacterial microcompartments are currently described, but only a few have both described – arrangement and 3D structure.
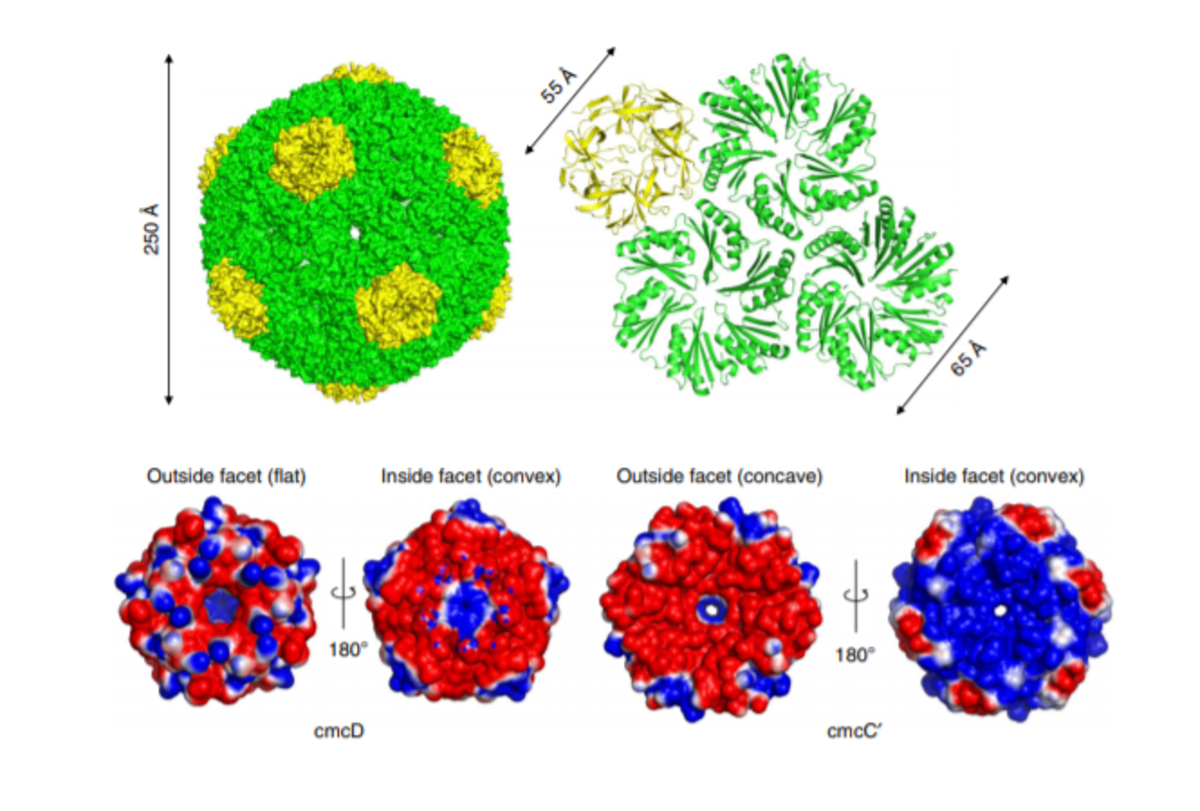
Choline lyase microcompartments in bacterium Klebsiella pneumonia were the main objects of the study. They are small protein capsules with a diameter of 25-50 nm. Just as a soccer ball is made up of regularly repeated pentagonal and hexagonal segments, the “shell” of the microcompartment is made up of repeatedly copied protein pentamers and hexamers. Inside the shell, in turn, is the main enzyme, choline lyase, and auxiliary enzymes, including alcohol dehydrogenase, acetaldehyde dehydrogenase and more. It turns out that these microcompartments can be “produced” by another bacterium common in molecular biology – Escherichia coli. However, enzymes do not always “self-assemble” in the expected microcompartment. The self-assembly of enzymes occurs in the presence of the “main” microlysing enzyme – choline lyase. By using cryoelectron microscopy and transmissive electron microscopy, it was possible to visualize the exact dimensions and external structure of the microcompartments. When the study was launched in early 2018, only one type of a bacterial microcompartment was known to the world, and the principles of how enzymes can self-assemble in these compartments were unknown.
An important step in the creation of specific biochemical systems
The team of UL and BMC researchers has provided valuable benefits in fundamental science. For the first time “Nature Communications” open access publication shows that the central enzyme, choline lyase, is the factor that provides the packaging for the other three enzymes. “Until now, the possibility that this is exactly how enzymes are packaged in microcompartments has been described theoretically, but it has not been shown in practice, that the packaging or encapsulation process is indeed characterized by a certain hierarchy. Namely, each enzyme has its own, inseparable role in a successful packaging process. We were also the first to observe a unique, hitherto uncharacteristic structure for the specific type of a microcompartment,” BMC research assistant, Dr.biol. Gints Kalniņš is satisfied, for whom the research is part of his doctoral thesis in the field of structural biology.
“If humanity were to learn how to replace enzymes in existing, naturally occurring bacterial microcompartments with others that are important in pharmacy or biotechnology, then these microcompartments would have a wide application in the synthesis of new substances or the degradation of toxic substances. By encapsulating or packing specific enzymes in microcompartments, stable biochemical systems could be obtained, which would realize only the reactions necessary for obtaining the product. To do this, we need to know the principles of microcompartment formation and enzyme incorporation. The completed research is an important step towards this goal, and the enzymes of interest could really be placed in the microcompartments,” explains J. Liepiņš.
The authors of the publication also include UL Faculty of Biology Masters student, BMC laboratory assistant and three-time UL Foundation’s scholarship holder Eva Emīlija Česle, BMC group leader, leading researcher Kaspars Tārs, researcher Juris Jansons, as well as a cooperation partner from University of Masarika in Brno, Czech Republic, Anatolijs Filimoņenko.
By implementing this cooperation project between the University of Latvia and BMC, supported by “Miktrotīkls”, the research team has discovered new opportunities for Latvian scientists to get involved in an internationally relevant and promising field of research. Furthermore, the team has succeeded in proving its scientific competence in this field by reporting the results in a high-quality journal with an impact factor of 11.8.

 Academic Centre
Academic Centre